Once a month, Joan Sirard puts up her feet, opens her iPad, and lets the IV in her arm deliver a promising new treatment for her early-stage Alzheimer’s disease.
She was diagnosed years ago after family members noticed a troubling pattern.
"Repeating. Repeating a lot. And they would say, ‘Ma, you just told us that,’ and I'd say, ‘I did?’", she explained.
“Because you don't remember."
Joan’s children found the Boston Center for Memory, in Newton, MA., and Joan enrolled in a clinic trial for the drug Aducanumab, which is aimed at people in the early stages of the disease.
"[Aducanumab] is aimed at removing the pathology from the brain that is killing the brain cells," said Dr. Paul Solomon, who works at the center. He said the drug works by removing amyloids, which are clumps of sticky proteins found in the brains of people with Alzheimer’s disease.
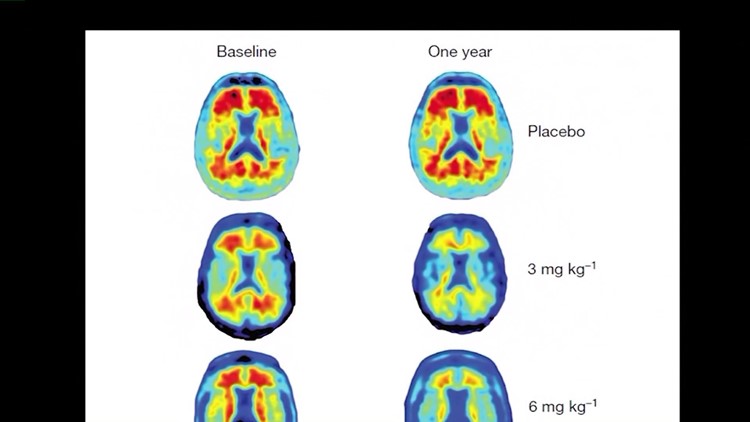
You can see the early results for yourself by looking at the results of a Phase I clinical trial that was published in the Journal “Nature.”
Brain scans of Alzehimer’s patients before the trial showed plenty of red blotches, which represent the amyloids.
After one year of the trial, patients who received a placebo – or no treatment – still had plenty of red blotches, but the patients who were given Aducanumab showed greatly reduced amounts of amyloids, and the higher the dosage, the less red their was.
In patients given the highest dosage, there were virtually no amyloids left.
"It was the first time that we had seen a combination of all of these things: that's removing pathology effectively combined with changes in cognition, memory, language and also changes in day-to-day functioning," said Solomon.
It’s an exciting find for both researchers and patients like Joan, who was lucky enough to be one of the patients in the double-blinded study who did receive the drug.
"I see the difference in me when I'm home and people have also said, a lot of people would say, 'no, you don't have Alzheimer's'," she said.
Currently, the trial has advanced to Phase III, in which researchers continue to check for efficacy at different dosages, and compare how the treatments do with other standard treatments.
Dr. Solomon said he expects the trial’s run of success to continue, and if it does, Aducanumab could be submitted to the Food and Drug Administration for approval, and be available for everyone.