HARTFORD — A Baltimore-based company has recalled some of its birth control pills due to the risk of contraceptive failure, according to the FDA.
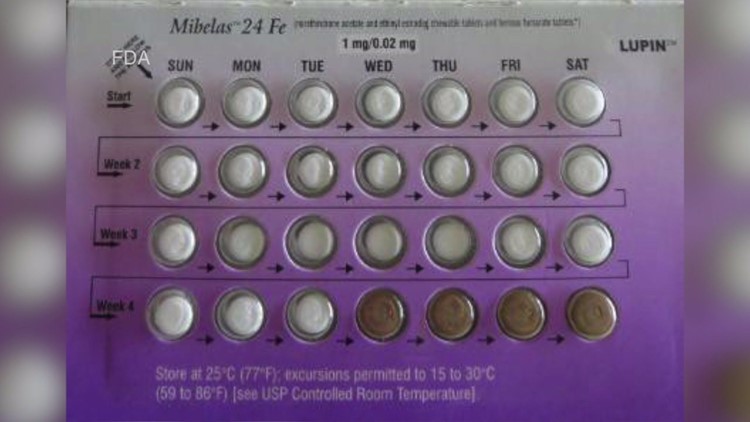
According to an FDA statement, Lupin Pharmaceuticals Inc., has recalled a specific lot of its Mibelas 24 Fe tablets. The lot number recalled has a number of L600518, with an expire date of May 31, 2018.
The recall stems from a complaint that indicated a packaging error has rotated the blister 180 degrees within the wallet, which reverses the weekly tablet orientation, and meant those on the first four days of therapy would have had the four non-hormonal placebo tablets, instead of the active tablets.
FDA said in result, oral contraceptive tablets that are taken out of sequence could place the user at risk of contraceptive failure and unintended pregnancy.