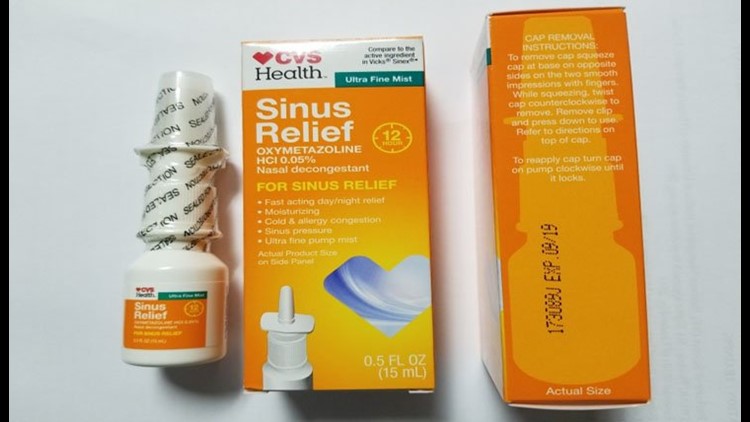
HARTFORD — CVS Health 12 Hour Sinus Relief Nasal Mist is being voluntarily recalled, according to the FDA. The product was found to have had microbiological contamination.
The nasal decongestant and is packaged in a 0.5 fluid ounce bottle that is placed in an individual folding carton. The affected CVS Health 12 Hour Sinus Relief Nasal Mist lot is Lot # 173089J, EXP 09/19.
Consumers with questions regarding this recall can call Product Quest Manufacturing LLC at (386) 239-8787, Monday through Friday from 8 am to 4 pm, EST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax: 1-800-FDA-0178.