FOX8 reports that the FDA has announced that a recall for medication used to treat high blood pressure has been expanded again.
According to the recall issued Wednesday, Legacy Pharmaceutical Packaging, LLC expanded its recall of 3 repackaged lots Losartan Tablets USP 50mg to include one additional lot. This recall was prompted due to Torrent Pharmaceuticals LTD issuing a Voluntary Nationwide Recall of Losartan Tablets, USP, due to the detection of trace amounts of N-Nitroso N-Methyl 4-amino butyric acid (NMBA) a possible process impurity or contaminant in an active pharmaceutical ingredient, manufactured by Hetero Labs Limited, (API manufacturer).
NMBA is a potential human carcinogen. To date, Legacy has not received any reports of adverse events related to this recall.
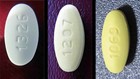
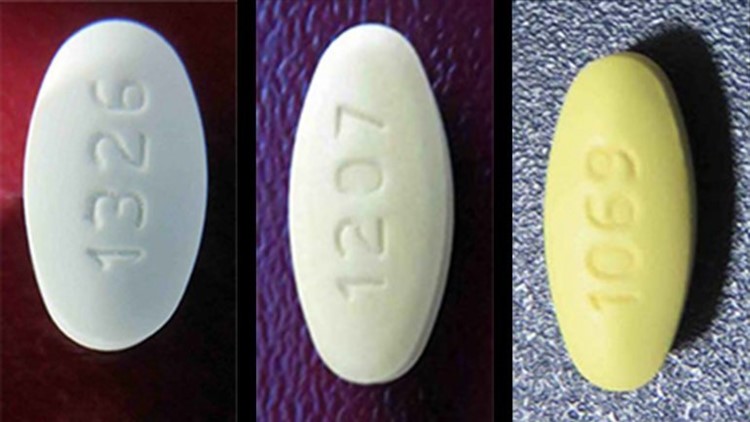
Losartan Potassium USP is a prescription medication used to treat high blood pressure and congestive heart failure and is packaged in 30 count bottles.
The identifying NDC number associated with Legacy’s product is as follows: Losartan Potassium, USP, 50mg NDC 68645-494-54
For complete details, click here.