WASHINGTON — U.S. regulators decided that Johnson & Johnson's single-dose vaccine provides enough protection against COVID-19 for nationwide use, with the FDA approving it for emergency use.
Trials found the shot to be 66% effective at preventing moderate to severe disease, 85% effective at preventing the most severe disease, and 100% effective at preventing hospitalizations and deaths as of 28 days post-vaccination.
This will be the third vaccine to hit the market in the U.S. Both Pfizer-BioNTech and Moderna's vaccines require two doses of an mRNA vaccine to reach their unprecedented high efficacy rates of about 95%.
Johnson & Johnson and AstraZeneca are studying a different kind of vaccine, called an adenovirus vector vaccine. It differs from the mRNA vaccines in a two key ways: the way it gets mRNA into your cells, and the way doses are stored and transported.
Here's everything you need to know about adenovirus vector vaccines:
SOURCES:
- Dr. Namandje Bumpus, Professor and Director of the Department of Pharmacology and Molecular Sciences at Johns Hopkins University
- Dr. William Moss, Executive Director of the International Vaccine Access Center and Professor of Infectious Disease Epidemiology, Molecular Microbiology and Immunology at Johns Hopkins University
How does the Johnson & Johnson vaccine work?
First, a recap of the vaccines we already have:
The mRNA vaccines work by directly injecting the genetic instructions for the spike protein on SARS-CoV-2, which is the virus that causes COVID-19.
When your immune system confronts that spike protein, your cells learn how to fight it off; this scrimmage prepares you to fight off COVID-19 should you ever come in contact with it.
You can watch a full mRNA vaccine explainer here:
Pfizer and Moderna's vaccines coat the mRNA in a sort of oily shell to get it into the cell. That's where the differences with the new vaccines begin.
Johnson & Johnson and AstraZeneca instead use a harmless host-virus to get into the cell. Their scientists alter the genetics of a harmless adenovirus to:
- Neutralize it so it cannot replicate in our cells, and
- Embed a DNA strand with the instructions to build the spike protein.
NOTE: Dr. William Moss says it is essential to note that while this vaccine uses the spike protein DNA, it is impossible for it to alter our human DNA. The COVID-19 vaccines will not alter any human genome and cannot make any changes to your DNA.

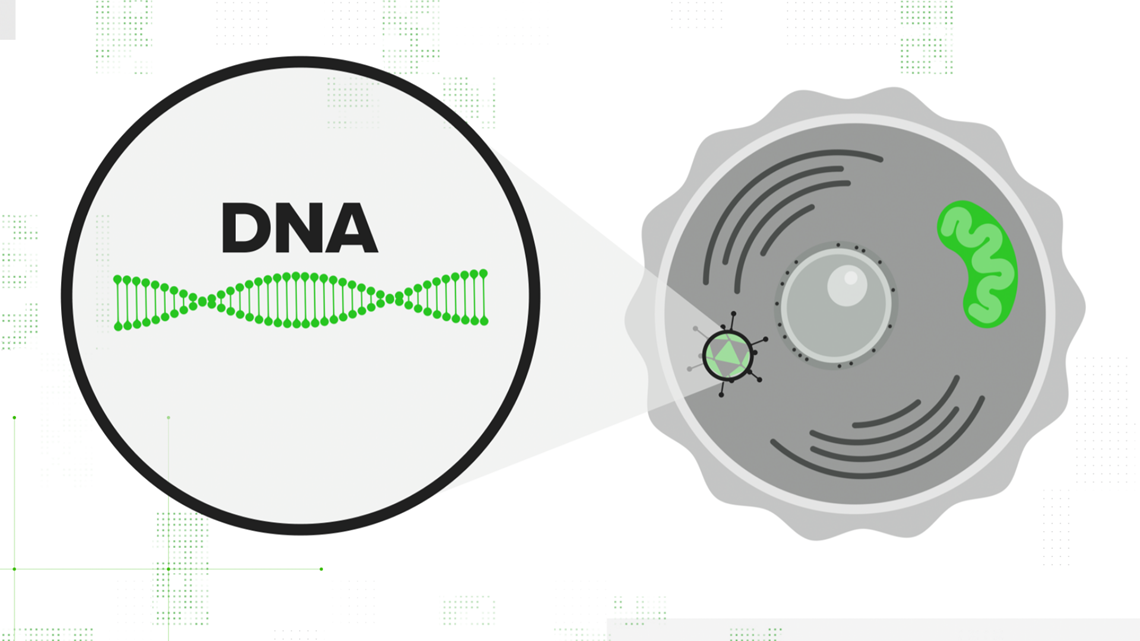

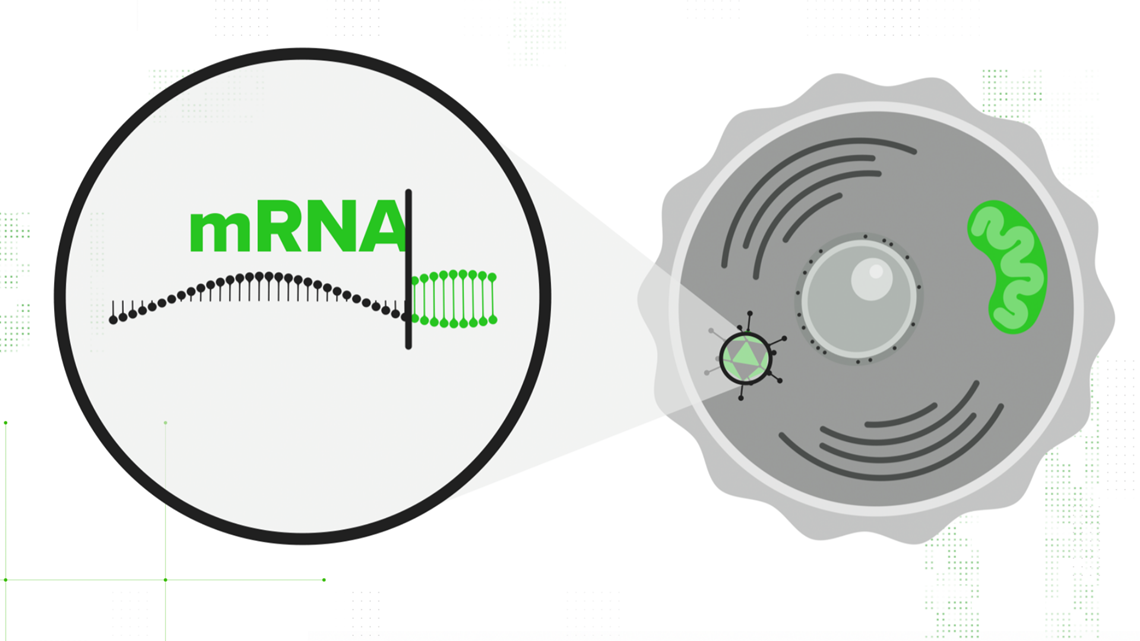
That adenovirus is injected into the cell, and the cell's nucleus accepts the DNA strand. Your cell translates that double-stranded DNA into mRNA — the same mRNA that the other vaccines start with. It contains all the instructions necessary to teach your cell how to build the spike protein. From there, the immunity process is the same.
Your cells use that mRNA to make SARS-CoV-2's signature spike protein, release it out into the body, and your immune system gets a practice round at fighting off COVID-19.

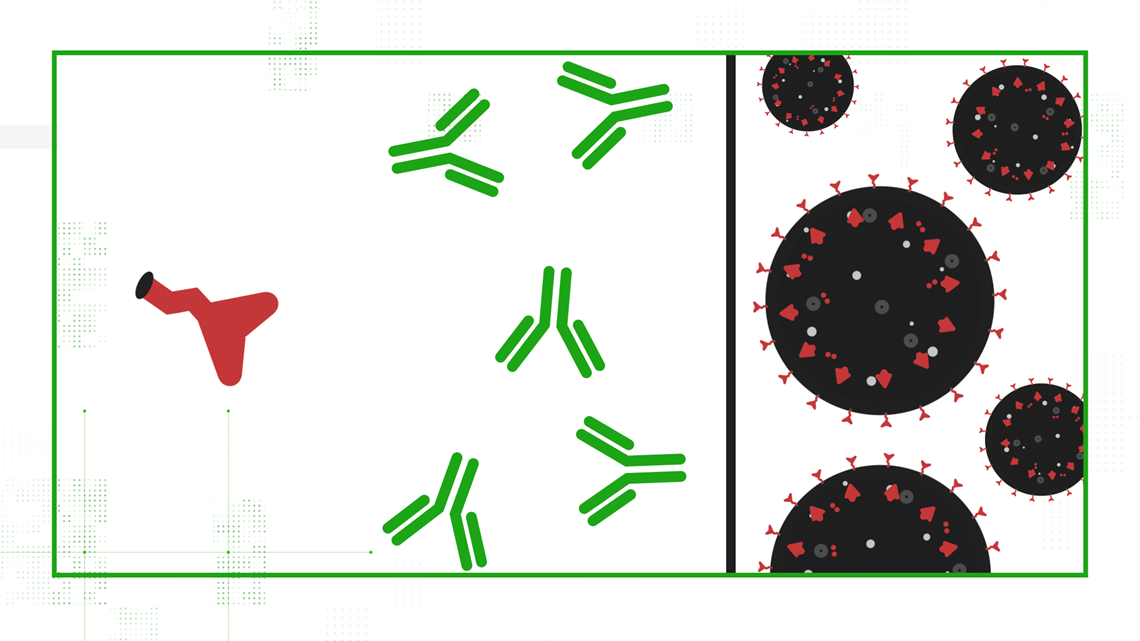
Johnson & Johnson's adenovirus vector vaccine requires just one dose to effectively teach your immune system how to fight off moderate to severe disease caused by COVID-19.
The company has two-dose trials currently ongoing but has not published any preliminary results as of the publishing of this story.
How are adenovirus vector vaccines stored and transported?
The rollout of the Pfizer and Moderna mRNA vaccines required ultra-cold storage — Pfizer's vaccine needs to be stored at -94 degrees Fahrenheit.
Many hospitals had to invest tens of thousands of dollars into CDC-recommended special freezers that could keep the vaccine doses from going bad. Dr. Moss says that's because mRNA is extremely heat sensitive.

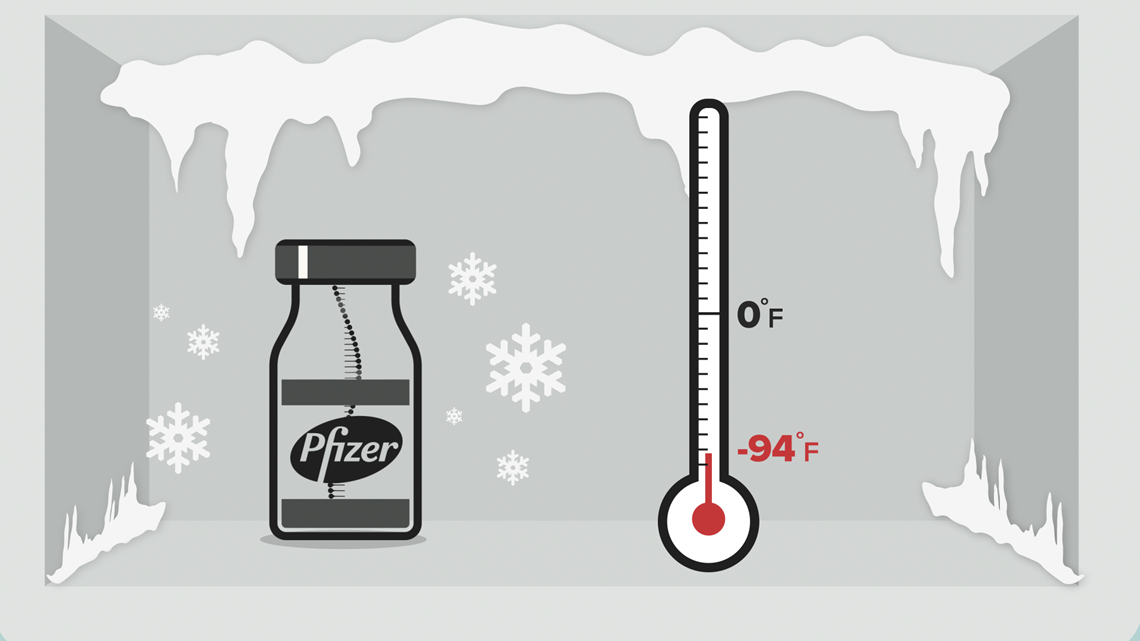

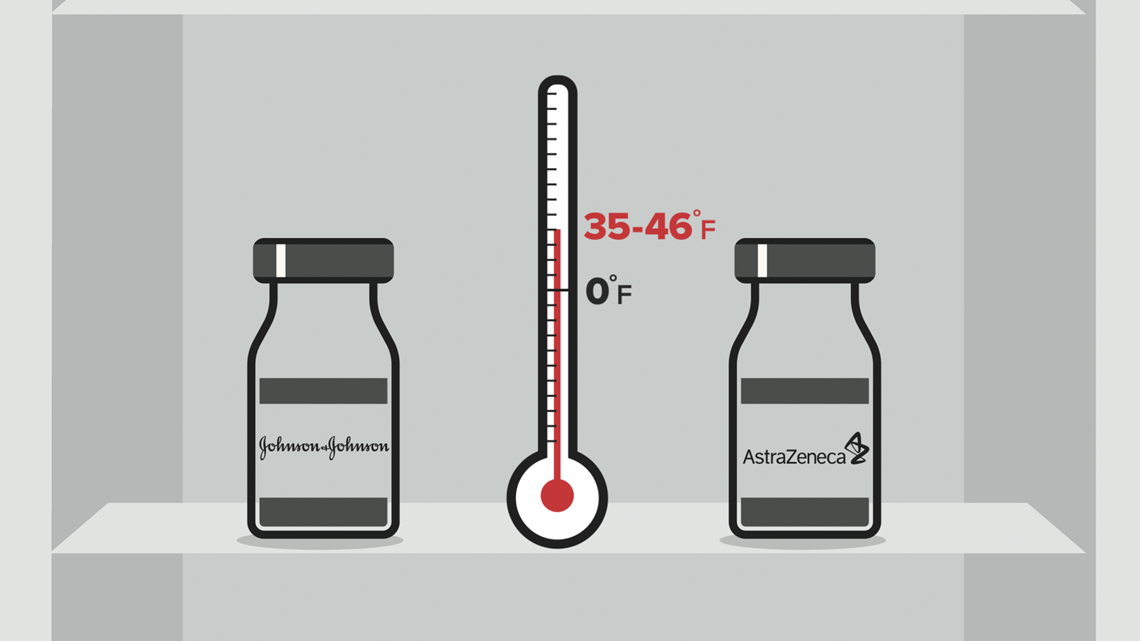
Johnson & Johnson and AstraZeneca's vaccines can be transported and stored for several months at normal refrigeration temperatures — between 35 and 46 degrees Fahrenheit. That makes distribution to places that can't afford extreme freezers a lot easier.
"The biggest impact of that is going to be in low and middle-income countries that can't afford -70 or -80 temperature freezers, but I think it'll even facilitate vaccine distribution in the United States," Dr. Moss explains. "It'll even be easier, let's say in rural parts of America, where it's just been hard to get the mRNA vaccines out."
The bottom line
While Johnson & Johnson's efficacy rate is lower than that of Pfizer and Moderna's, 72% in the US to their 95%, experts believe it still has a key role in getting us to the light at the end of the tunnel.
Dr. Anthony Fauci told Verify's Gabe Cohen in January that despite a lower number on paper, the 100% effectiveness of preventing hospitalizations and deaths will save a lot of people.
"Practically speaking," Dr. Fauci says, "if the only thing you're worried about is keeping people out of the hospital and not getting people seriously ill, there clearly is value-added with the Johnson and Johnson [vaccine]."


